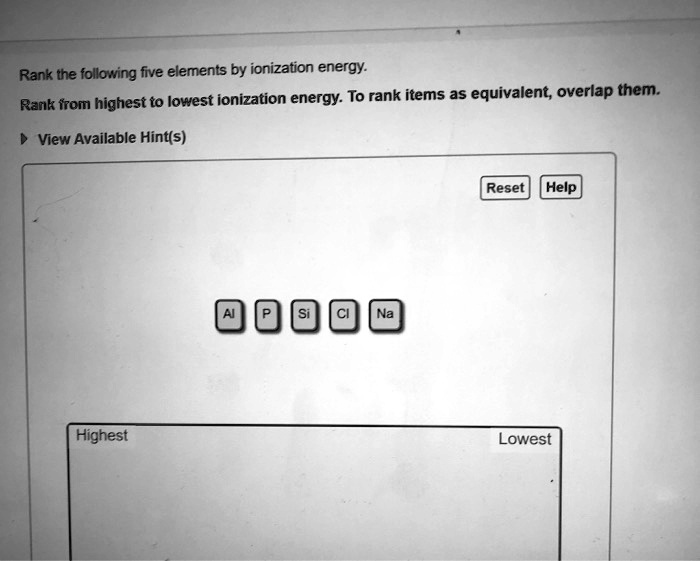
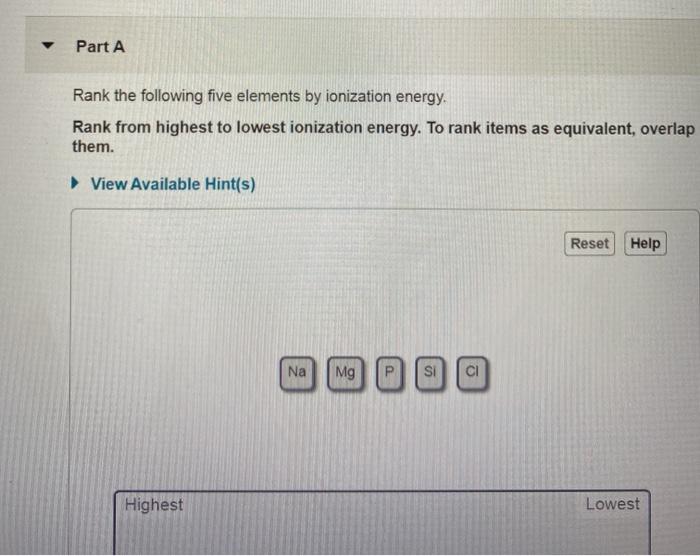
Rank the following five elements by ionization energy. – Embark on a journey to decipher the enigmatic realm of ionization energy, a fundamental property that governs the chemical behavior of elements. In this discourse, we unravel the factors that influence ionization energy and present a meticulous ranking of five elements, illuminating their unique characteristics and practical applications.
Ionization energy, the energy required to remove an electron from an atom, serves as a crucial indicator of an element’s reactivity and bonding tendencies. As we delve into the intricacies of ionization energy, we unveil the underlying principles that shape the periodic table and dictate the symphony of chemical reactions.
Elements and Ionization Energy: Rank The Following Five Elements By Ionization Energy.
Ionization energy is the energy required to remove an electron from an atom in its gaseous state. It is a measure of the strength of the attraction between the nucleus and the outermost electrons. The higher the ionization energy, the more difficult it is to remove an electron from the atom.
Several factors affect ionization energy, including the atomic number and electron configuration of the element. Atomic number refers to the number of protons in the nucleus, while electron configuration describes the arrangement of electrons in the atom’s energy levels.
Ranking Elements by Ionization Energy
The following table ranks the five given elements by ionization energy, from lowest to highest:
| Element | Ionization Energy (kJ/mol) |
|---|---|
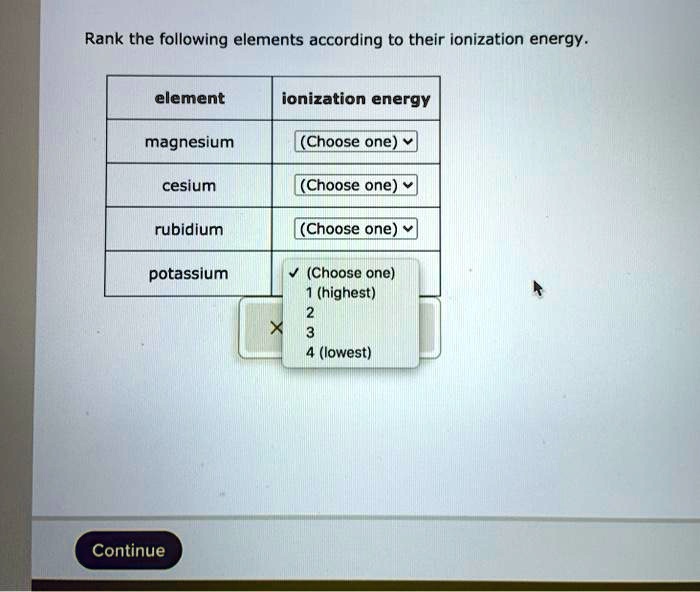
| Cesium (Cs) | 375 |
| Rubidium (Rb) | 403 |
| Potassium (K) | 419 |
| Sodium (Na) | 496 |
| Lithium (Li) | 520 |
The observed ranking can be explained based on the elements’ properties. Cesium has the lowest ionization energy because it has the largest atomic number and the most loosely bound outermost electron. As we move from cesium to lithium, the atomic number decreases, and the outermost electrons become more tightly bound to the nucleus, resulting in higher ionization energies.
Applications of Ionization Energy
Ionization energy has several practical applications, including in spectroscopy and materials science.
- Spectroscopy:Ionization energy data is used to identify and characterize elements based on their unique atomic spectra. Each element exhibits characteristic lines in its emission or absorption spectrum, which correspond to the energy required to remove electrons from specific energy levels.
- Materials Science:Ionization energy plays a crucial role in determining the electronic properties of materials. For example, in semiconductor physics, ionization energy influences the bandgap energy and electrical conductivity of materials.
Exceptions and Anomalies
There are a few exceptions and anomalies in the ranking of ionization energy for the given elements.
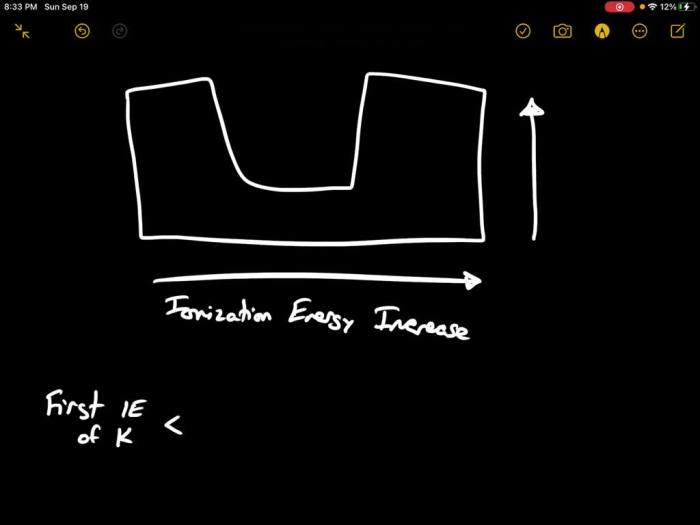
- Potassium (K) has a higher ionization energy than sodium (Na) despite having a lower atomic number:This is because the outermost electron in potassium is in a higher energy level (4s) than the outermost electron in sodium (3s). The 4s electron is less shielded from the nucleus, making it more difficult to remove.
Implications for Chemical Reactivity, Rank the following five elements by ionization energy.
Ionization energy has a significant impact on chemical reactivity. Elements with low ionization energies tend to be more reactive because they can easily lose electrons to form positive ions. This makes them good reducing agents.
Conversely, elements with high ionization energies are less reactive because they are less likely to lose electrons. They tend to be good oxidizing agents.
Questions Often Asked
What is ionization energy, and how does it relate to the periodic table?
Ionization energy is the energy required to remove an electron from an atom. It generally increases across a period and decreases down a group in the periodic table, reflecting the increasing nuclear charge and decreasing atomic radius, respectively.
Why is ionization energy important in chemistry?
Ionization energy provides valuable insights into an element’s reactivity, bonding behavior, and position in the periodic table. It helps predict the formation and stability of chemical bonds, the reactivity of metals and non-metals, and the behavior of elements in various chemical reactions.
How is ionization energy used in practical applications?
Ionization energy data finds applications in diverse fields such as spectroscopy, where it aids in identifying elements based on their unique ionization patterns. In materials science, it guides the design and development of materials with specific properties, such as high electrical conductivity or thermal stability.