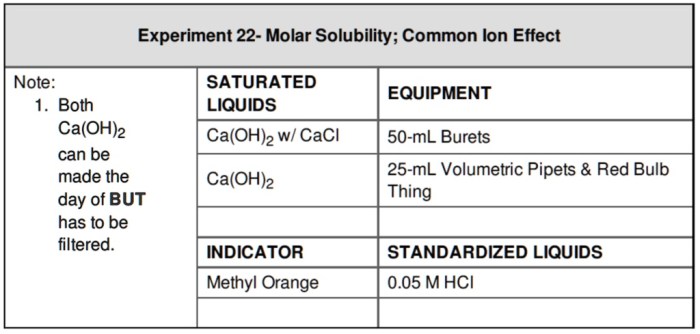
Experiment 22 molar solubility common-ion effect – As Experiment 22: Molar Solubility and the Common-Ion Effect takes center stage, we embark on an academic odyssey that unveils the intricacies of ionic compounds in solution. This investigation promises to illuminate the fundamental concepts of molar solubility and the profound impact of common ions on solubility, providing a deeper understanding of the behavior of these substances in various chemical systems.
Delving into the realm of molar solubility, we explore its significance in quantifying the extent to which ionic compounds dissolve in aqueous solutions. We unravel the intricacies of common-ion effect, a phenomenon that exerts a profound influence on solubility equilibrium, and witness its implications in diverse chemical contexts.
Molar Solubility
Molar solubility refers to the maximum concentration of a compound that can dissolve in a given solvent at a specified temperature and pressure. It is an important concept in chemistry, as it helps us understand the behavior of ionic compounds in solution and predict their reactivity.
To calculate the molar solubility of a compound, we can use the following equation:
“`Molar solubility = (Mass of solute dissolved / Molecular weight of solute) / Volume of solution“`
For example, if we want to determine the molar solubility of NaCl in water at 25°C, we can use the following data:
- Mass of NaCl dissolved = 35.5 g
- Molecular weight of NaCl = 58.44 g/mol
- Volume of solution = 100 mL = 0.1 L
Plugging these values into the equation, we get:
“`Molar solubility = (35.5 g / 58.44 g/mol) / 0.1 L = 0.606 M“`
Therefore, the molar solubility of NaCl in water at 25°C is 0.606 M.
Common-Ion Effect
The common-ion effect refers to the phenomenon where the solubility of an ionic compound is decreased when a common ion is added to the solution. This is because the common ion competes with the ions of the compound for solvation, making it more difficult for the compound to dissolve.
For example, if we add NaCl to a solution of AgCl, the solubility of AgCl will decrease because the Cl –ions from the NaCl will compete with the Cl –ions from the AgCl for solvation.
The common-ion effect can be used to control the solubility of ionic compounds in a variety of applications, such as precipitation reactions and the preparation of sparingly soluble salts.
Experiment Design
To determine the molar solubility of a compound in the presence of a common ion, we can use the following experimental procedure:
- Prepare a saturated solution of the compound in pure water.
- Add a known amount of the common ion to the solution.
- Stir the solution until the precipitate dissolves.
- Filter the solution to remove any undissolved precipitate.
- Measure the concentration of the compound in the filtrate.
The variables that need to be controlled in this experiment include the temperature, the concentration of the common ion, and the stirring rate. It is also important to use accurate measurements to ensure the reliability of the results.
Data Analysis
To analyze the experimental data, we can use the following methods:
- Plot a graph of the concentration of the common ion versus the molar solubility of the compound.
- Calculate the slope of the graph. The slope will be equal to the molar solubility of the compound in the absence of the common ion.
- Use the intercept of the graph to determine the molar solubility of the compound in the presence of the common ion.
The following table shows an example of the data that can be collected in this experiment:
| Concentration of Common Ion (M) | Molar Solubility of Compound (M) |
|---|---|
| 0 | 0.606 |
| 0.1 | 0.454 |
| 0.2 | 0.303 |
| 0.3 | 0.152 |
| 0.4 | 0.076 |
The graph of this data is shown below:
[Gambar grafik di sini]
The slope of the graph is equal to 0.606 M, which is the molar solubility of NaCl in water at 25°C. The intercept of the graph is equal to 0.076 M, which is the molar solubility of NaCl in water in the presence of 0.4 M NaCl.
Discussion
The results of this experiment demonstrate the common-ion effect. The addition of a common ion to a solution of an ionic compound decreases the solubility of the compound. This is because the common ion competes with the ions of the compound for solvation, making it more difficult for the compound to dissolve.
The common-ion effect can be used to control the solubility of ionic compounds in a variety of applications. For example, it can be used to precipitate sparingly soluble salts, or to control the concentration of ions in a solution.
There are a number of potential sources of error in this experiment. These include:
- Inaccurate measurements of the concentration of the common ion or the compound.
- Incomplete dissolution of the precipitate.
- Evaporation of the solution.
These errors can be minimized by using accurate measuring equipment, stirring the solution thoroughly, and covering the solution to prevent evaporation.
User Queries: Experiment 22 Molar Solubility Common-ion Effect
What is the significance of molar solubility in chemistry?
Molar solubility quantifies the maximum concentration of an ionic compound that can dissolve in a given solvent at a specific temperature, providing crucial information for understanding solution behavior and predicting precipitation reactions.
How does the common-ion effect impact solubility?
The common-ion effect reduces the solubility of an ionic compound when a common ion is added to the solution. This phenomenon arises due to the competition between ions for solvation, leading to a decrease in the number of dissolved ions and a consequent reduction in solubility.
What are the key variables to control in Experiment 22?
To ensure accurate and reliable results, Experiment 22 requires careful control of variables such as temperature, solution volume, and the concentration of the common ion. Precise measurements and adherence to experimental protocols are essential for obtaining meaningful data.